Popular local and semilocal density functionals are unable to describe correctly van der Waals interactions resulting from dynamical correlations between fluctuating charge distributions. Typically this is done by applying the ideas of quantum mechanics to molecules and RayleighSchrödinger perturbation theory has been especially effective in this regard.
Difference Between Van Der Waals And Hydrogen Bonds
Why Do Van Der Waals Forces Hold Molecules Together Socratic

Ib Chemistry Standard Level Notes Intermolecular Forces
An explanation of how hydrogen bonding arises and its effect on boiling points.

Van der waals forces examples. The Mo 2 C strongly coordinates with CN to endow CN ordered in-plane electron migration. There are two types of Van der Waals forces which we will discuss below London dispersion forces and dipole-dipole forces interactions. The Van Der Waals equation for non-ideal gases takes into consideration these intermolecular forces.
However ethanol has a hydrogen atom attached directly to an oxygen. It is about 120th 5 the strength of the covalent bond formed between O-H. These forces determine whether a substance is a solid liquid or gas at a given temperature.
Dispersion forces for example were described by. Van der Waals force is a general term that describes any attractive intermolecular force between molecules and includes both the London dispersion force and the dipole-dipole force discussed. When a very smooth object such as cutlery is applied to very smooth skin Van der Waals forces can bond it to the body especially if there is a bit of oil or moisture present since both of these can engage in Van der Waals bonding.
The term van der Waals interaction has become effectively meaningless because definitions are so inconsistent and arbitrary and because it does not describe interactions in a physically meaningful way. The g-C 3 N 4 and Mo 2 C based 2D2D novel Van der Waals heterostructure is reported. However even this weak bond is strong enough to withstand slight temperature fluctuation.
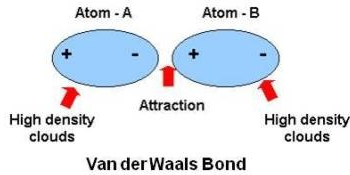
The McLachlan theory predicts that van der Waals attractions in media are weaker than in vacuum and follow the like dissolves like rule which means that different types of atoms interact more weakly than identical types of. The internal electric field is established and it synergistically enhances the photocatalytic performance. Van der Waals forces are the weak forces that contribute to intermolecular bonding between moleculesMolecules inherently possess energy and their electrons are always in motion so transient concentrations of electrons in one region or another lead electrically positive regions of a molecule to be attracted to the electrons of another molecule.
These London dispersion forces are often found in the halogens eg F 2 and I 2 the noble gases eg Ne and Ar and in other non-polar molecules such as carbon dioxide and methane. Van der Waals forces. It represents the distance of the closest approach for another atom.
The bonds get firmer when they occur with a short distance from 04 kilojoules per mole kJmol to 4 kJmol. There doesnt seem to be any intra molecular hydrogen bonds either. Additionally with fully saturated chemical bonds on the surface the interactions between neighbouring layers of 2DLMs are usually characterized by van der Waals forces.
These forces arise from the interactions between uncharged atomsmolecules. Van synonyms van pronunciation van translation English dictionary definition of van. Van der Waals radius is the radius of an imaginary sphere surrounding an atom.
Van der Waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. A more general theory of van der Waals forces in condensed media was developed by A. Alternatively van der Waals vdW integration in which pre-fabricated building blocks are physically assembled together through weak vdW interactions offers an alternative low-energy material.
The van der Waals attractions both dispersion forces and dipole-dipole attractions in each will be similar. There are intermolecular forces between neutral non-polar atoms called London dispersion Van der Waals interactions. Since this entry has the largest number of atoms it.
Van der Waals forces disappear as the distances between the atoms or molecules increase 1-7. McLachlan in 1963 and included the original Londons approach as a special case. A description of van der Waals forces temporary fluctuating dipole and dipole-dipole interactions causing attractions between individual molecules.
This deviation from the ideal gas properties can be explained by van der Waals equation given below which takes into account the volume occupied by the molecules of gas and also the force of attraction that may exist between them ie the van der Waals forces. 916 These series of attractions and repulsions form as a consequence of polarization fluctuation between neighboring particles. The Van Der Waals forces appear as an interaction in a closely-situated position of the molecules or atoms.
It is due to van der Waals forces that real gases deviate from their ideal gas properties. In general all the intermolecular forces of attraction between molecules are called Van der Waals forces. A hydrogen bond tends to be stronger than van der Waals forces but weaker than covalent bonds or ionic bonds.
An enclosed boxlike motor vehicle having rear or side doors and side panels especially for. Van der Waals forces can be classified as weak London dispersion Forces and stronger dipole-dipole forces. For example Van der Waals forces can arise from the fluctuation in the polarizations of two particles that are close to each other.
London dispersion forces are part of the van der Waals forces or weak intermolecular attractions. 9 Though still weaker than H-binding hydrophobic attraction and ionic. Molecular interactions are also known as noncovalent or intermolecular or non-bonding or van der Waals interactions or noncovalent or intermolecular or non-bonding forces.
Advanced characterizations combined with DFT calculations confirm the strong electronic coupling. A simple explanation of the forces holding metals together. Such a syllabus will talk about van der Waals forces meaning dispersion forces and separately dipole-dipole interactions.
I am assuming that you mean the inter-molecular forces between two hexane molecules. Answer 1 of 7. This also explains why flies can walk on ceilings.
Here the oxygen still has two lone pairs like a water molecule. Both of these forces are due to momentarily dipole formation. London Dispersion Forces vs Van der Waals Forces.
The various different types were first explained by different people at different times. A pragmatic method to work around this problem is to add a correction to the conventional Kohn-Sham DFT energy E K S D F T displaystyle E_mathrm KS-DFT. These forces depend on the attractions or repulsions within two or more molecules.
Theres no hydrogen bonding between these molecules so they have normal interactions based on their molecular properties. Alternatively one may seek a fundamental unifying theory that is able to explain the various types of interactions such as hydrogen bonding van der Waals forces and dipoledipole interactions. All intermolecular attractions are known collectively as van der Waals forces.
Van der Waals forces are a group of interactions between atoms andor within individual molecules that take place based upon the interaction of electron clouds surrounding two polar systems.

Van Der Waals Forces Chemistry And Physics Britannica

Van Der Waals Forces Chemistry For Non Majors

Van Der Waals Forces Overview Role In Biology Expii
1

How Van Der Waals Forces Work Youtube

Van Der Waals Forces Bond Definition Examples Diagrams

Van Der Waals Forces Chemistry For Non Majors

Direct Measurement Of Van Der Waals Force Made For The First Time