The reaction also releases heat but it usually occurs too slowly for this to be noticeable. HClaq NaOHaq NaClaq HOHl Types of Decomposition Reaction.

What Is Double Decomposition Reaction Related Writing And Balancing Of Chemical Equations Edurev Class 10 Question

What Is A Decomposition Reaction Definition And Examples

Breakage Of Bonds Formation Of Bonds
The burning of.

Double decomposition reaction. Scavengers slow down the chain-reaction. 232 suggest appropriate practical methods to measure the rate of a reaction and collect reliable data methods limited to measuring a change in mass gas volume or formation of a precipitate against time for the reaction of. Zn 2HCl ZnCl2 H2 Select one.
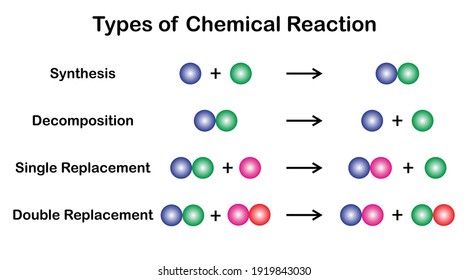
None of above d. Na 3 PO 4 3 KOH 3 NaOH K 3 PO 4 double displacement 8. A reaction between two compounds in which parts of each compound is interchanged in order to form two new compounds is called a double decomposition reaction.
An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. A decomposition reaction is a chemical reaction in which some chemical bonds in a compound are broken and simpler substances are formed. In a synthesis reaction there are _____ two or more products.
Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and. A chemical reaction in which compounds break up into simpler constituents is a _____ reaction. If no acid or base the decomposition does not take place.
Further questions just have the reactants listed and you should decide on. Single replacement Complete and balance the. 2 H2O 2 H2 O2 16.
A double decomposition reaction is a type of decomposition reaction in which two constituent reactants interchange positive and negative ions and form two new compounds. In double replacement both reactants are compounds each with a cation part and an anion part. Classify the following reaction.
A decomposition reaction _____ requires energy in. The breaking of chemical bonds requires the addition of energy usually in the form of heat. 4Fe 3O 2 2Fe 2 O 3 ΔH - 1648 kJmol.
Decomposition Reaction The types of reaction in which a single reactant breaks down to give simpler products are called decomposition reaction. Calcium oxide must have the formula CaO because calcium forms an ion with a 2 charge. Decomposition reactions are initiated by the addition of energy.
Decomposition single replacement and double replacement and combustion reactions. Decomposition reactions are also known as analysis reactions or chemical breakdowns. Double displacement Identify each type of reaction.
A reaction between two compounds where the positive ion of one compound is exchanged with another compounds positive ion is called a double replacement. An addition reaction is essentially a reverse decomposition reaction wherein a decomposition reaction is a reaction where one compounds one or more elements or compounds. A double replacement reaction is called a double decomposition reaction but the term is reserved for when one or both of the reactants doesnt dissolve in a solvent.
Combustion the thermite reaction combining strong acids and bases polymerizationsAs an example in everyday life hand warmers make use of the oxidation of iron to achieve an exothermic reaction. A particularly important class of exothermic reactions is combustion of a hydrocarbon fuel eg. See practice problem 6 for an example.
Pb FeSO 4 PbSO 4 Fe single displacement 11. Electrochemical reactions are redox oxidation and reduction reactions that convert chemical energy into electrical energy. Combination A chemical reaction in which compounds break up into simpler constituents is a decomposition reaction.
But only chemical compounds containing multiple bond character can undergo an addition reaction as a double or triple bond is usually broken to form the required single bonds. The addition of carbonate CO 3 2- can increase the half-life of ozone 56. Carbonate compounds will decompose when heated.
There is another example in the 10 problems but youll have to figure out which one. Potassium permanganate is an inorganic compound with the chemical formula KMnO 4 and composed of K and MnO 4It is a purplish-black crystalline salt that dissolves in water to give intensely pink or purple solutions. For the first few reactions the type of reaction is listed you should predict the products then balance.
Double Replacement Reaction Examples. Here is the chemical equation for the rusting of iron. In chemistry there are two main types of changes - physical changes and chemical changes.
A double displacement reaction is also called a double replacement reaction salt metathesis reaction or double decomposition. 23 Rates of reaction. Carbonate is a scavenger with a strong effect.
CaCO_3s - CaOs CO_2g Calcium carbonate has a 11 ratio because calcium forms a 2 ion and carbonate has a -2 charge. Physical changes affect the shape size and form of a substance. MgCl 2 Li 2 CO 3 MgCO 3 2 LiCl double displacement 9.
C 6 H 12 9 O 2 6 CO 2 6 H 2 O combustion 10. Here is the balanced equation for this reaction. Think of double replacement chemical reactions or metathesis reactions like a chemical trade.
Metals with dilute acid CCEA Double award science. Calcium carbonate will decompose to form carbon dioxide and calcium oxide. CaCO 3 CaO CO 2 decomposition 12.
In a combustion reaction oxygen is _____ on the reactant side. Examples of Double Replacement Chemical Reactions. A decomposition reaction is one where a molecule breaks apart into simpler ones.
Also called a double replacement reaction this type of reaction occurs when the cations of two chemical compounds switch places. Eg- When a decomposition reaction is carried out by heating it is known as thermal decomposition. P 4 3 O 2 2 P 2 O 3 synthesis 13.
When you have two complex reactants AB and CD that swap chemicals during a reaction you get two new products AC and BD. This is because after the reaction of scavengers with OH-radicals the reaction products do not react with ozone any further. The opposite of this type of reaction is a synthesis in which simpler reactants combine to form a more complex product.
Fe O 2 H 2 O Fe 2 O 3. The reaction occurs most often between ionic compounds although technically the bonds formed between the chemical species may be either ionic or covalent in nature.

Double Decomposition Reactions Lessons Blendspace

Introduction To Double Decomposition Reaction Youtube
Why Is A Double Displacement Reaction Also Called A Double Decomposition Reaction Quora

Introduction Of Decomposition Reaction In Chemistry Aesl

Difference Between Double Displacement And Double Decomposition Reaction Compare The Difference Between Similar Terms

Reactions Types
Is It Necessary That All Precipitation Reaction Is A Double Displacement Reaction Quora
Chemical Decomposition Images Stock Photos Vectors Shutterstock